Volunteers Needed for Medical Study
[caption id="attachment_1183" align="alignnone" width="488"]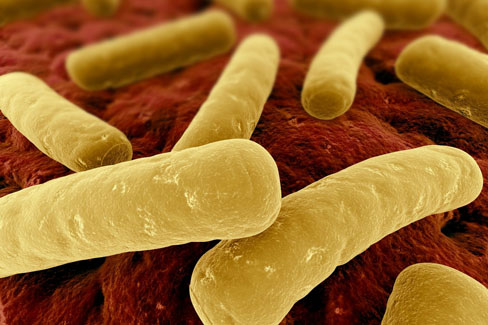
Sarasota has been named one of the first sites out of 200 nationally to participate in a study, fast-tracked by the FDA, to test the efficacy of a new vaccine for C. diff, a dangerous hospital-acquired superbug.
Called "C.diffense" and headed locally by Dr. Michael Swor, the phase III clinical trial needs volunteers to participate.
The vaccine is one of the first of its kind and may represent a major breakthrough in the use of vaccines to combat the superbugs that have arisen from overuse of antibiotics. Because of the urgent need to combat C. diff, the FDA is expediting the process of approving the drug, but how quickly that happens "depends on how quickly we can get people into the study and collect the required data," says Swor. "We need more participants. This could save lives."
Visit the study's website here to find out about your eligibility and other information.



